How Propofol is Broken Down in the Body
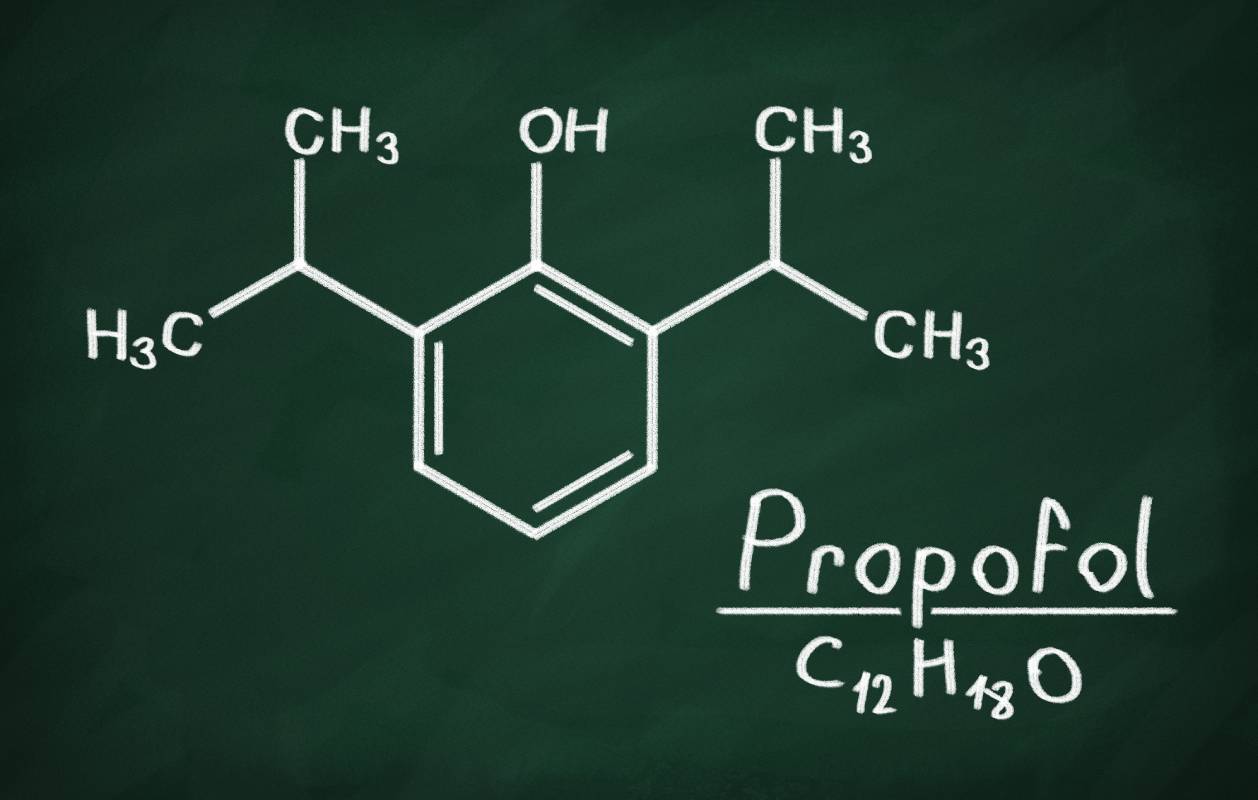
Propofol is an intravenous anesthetic agent commonly used for procedural anesthesia, in which a patient is given a sedating medication along with an analgesic medication that enable them to tolerate painful procedures.1 It is also used to induce general anesthesia, and is therefore sometimes referred to as an “induction agent.”2 The propofol molecule is an alkylphenol: the molecule contains a benzene ring – six carbons arranged in a hexagon – attached to an oxygen and hydrogen atom, which is known as the hydroxyl group. The medication was discovered in 1977 and approved by the FDA in 1989.3
Propofol, like most drugs, is metabolized, or chemically broken down, in the body before excretion. Understanding how and when a drug is metabolized is critical for those prescribing drugs, as drug metabolism impacts how long a drug can remain effective and under which conditions a drug might become hazardous. Propofol is mainly metabolized in the liver, where the drug is converted to inactive, water-soluble metabolites, but it can be cleared in other parts of the body: the brain, kidney, and small intestine are also sites of propofol metabolism.4
The propofol molecule is broken down by a class of enzymes known as uridine glucuronosyltransferases, or UGTs. These enzymes catalyze metabolism through a chemical process known as glucuronidation, in which a molecule containing the glucuronic acid component is added to and deactivates a compound. In the case of propofol, this occurs via a multi-step chemical reaction involving propofol’s hydroxyl group and several of its carbon atoms.5 UGT1 is the main enzyme responsible for propofol metabolism, followed by CYP2B6 and SULT1A.6
Propofol metabolites are eliminated from the body in urine after being broken down. Simons et al. found that less than 1% of administered propofol is excreted in the urine without being broken down, while only 2% is excreted in feces, which indicates that propofol metabolism is highly efficient.7 An interesting and rare side effect of propofol is urine discoloration. In the several reported cases of this side effect in literature, patients describe passing green, pink, or white urine.5 Urine discoloration is harmless and not toxic to the kidneys. It is not fully understood why this can occur, but researchers suspect that is caused by certain compounds that form from propofol metabolism.5
Phenol-containing drugs often cause pain on injection, but injections of propofol are notoriously painful. Approximately 70% of patients who receive a propofol injection, without first receiving a pretreatment, report pain following injection.8 To bypass this issue, some anesthesiologists prefer to use fospropofol, a derivative of propofol that is less painful on injection and also carries a lower risk of bacterial contamination.9 Though the fospropofol molecule differs from the propofol molecule only in the presence of a phosphate group – a phosphorous atom bound to four oxygen atoms – instead of the hydroxyl group, this is enough to dramatically alter its metabolism. Fospropofol is metabolized by an enzyme called 10-formyltetrahydrofolate dehydrogenase into carbon dioxide and water, among other compounds.5 Though propofol cannot be taken orally, fospropofol can and provides propofol bioavailability when administered orally, an interesting result of its metabolism.
References
1. Benzoni, T. & Cascella, M. Procedural Sedation. in StatPearls (StatPearls Publishing, 2022).
2. Procedural Sedation. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/procedural-sedation (2019).
3. Hofschneider, M. Discovery and development of propofol, a widely used anesthetic. Lasker Foundation https://laskerfoundation.org/winners/discovery-and-development-of-propofol-a-widely-used-anesthetic/.
4. Zhang, S. H., Li, Q., Yao, S. L. & Zeng, B. X. Subcellular expression of UGT1A6 and CYP1A1 responsible for propofol metabolism in human brain. Acta Pharmacol. Sin. 22, 1013–1017 (2001), PMID: 11749793
5. Dinis-Oliveira, R. J. Metabolic Profiles of Propofol and Fospropofol: Clinical and Forensic Interpretative Aspects. BioMed Res. Int. 2018, 6852857 (2018), DOI: 10.1155/2018/6852857
6. Martin, Y. N. & Nicholson, W. T. Chapter 58 – Pharmacogenomics. in Essentials of Neuroanesthesia (ed. Prabhakar, H.) 913–925 (Academic Press, 2017). doi:10.1016/B978-0-12-805299-0.00058-0, https://doi.org/10.1016/B978-0-12-805299-0.00058-0
7. Simons, P. J. et al. Disposition in male volunteers of a subanaesthetic intravenous dose of an oil in water emulsion of 14C-propofol. Xenobiotica Fate Foreign Compd. Biol. Syst. 18, 429–440 (1988), DOI: 10.3109/00498258809041679
8. Kang, H.-J. et al. Clinical factors affecting the pain on injection of propofol. Korean J. Anesthesiol. 58, 239–243 (2010), doi: 10.4097/kjae.2010.58.3.239
9. Feng, A. Y., Kaye, A. D., Kaye, R. J., Belani, K. & Urman, R. D. Novel propofol derivatives and implications for anesthesia practice. J. Anaesthesiol. Clin. Pharmacol. 33, 9–15 (2017), DOI: 10.4103/0970-9185.202205
